Imagine this scenario: you’re dying of thirst, and all you can think about is quenching it with a tall glass of ice-cold water.
But what if that seemingly harmless drink was spiked with chloroform? Suddenly, the idea of taking a sip becomes terrifying.
You may be thinking, “Is this some twisted plot from a horror movie?” Unfortunately, the use of chloroform in water is not just a figment of our imagination; it has happened in the past for various reasons.
But what exactly happens when you add chloroform to water? And should we be concerned about its impact on our health and the environment?
So grab your favorite beverage (sans chloroform), sit back, and join me as we explore this controversial topic together.
What Happens If You Put Chloroform In Water?
Contents
- 1 What Happens If You Put Chloroform In Water?
- 2 The Chemical Properties of Chloroform
- 3 The Effects of Adding Chloroform to Water
- 4 Environmental Impact of Chloroform in Water
- 5 Health Hazards of Chloroform Exposure
- 6 Risks for Aquatic Life from Chloroform in Water
- 7 Proper Disposal Methods for Chloroform
- 8 Conclusion
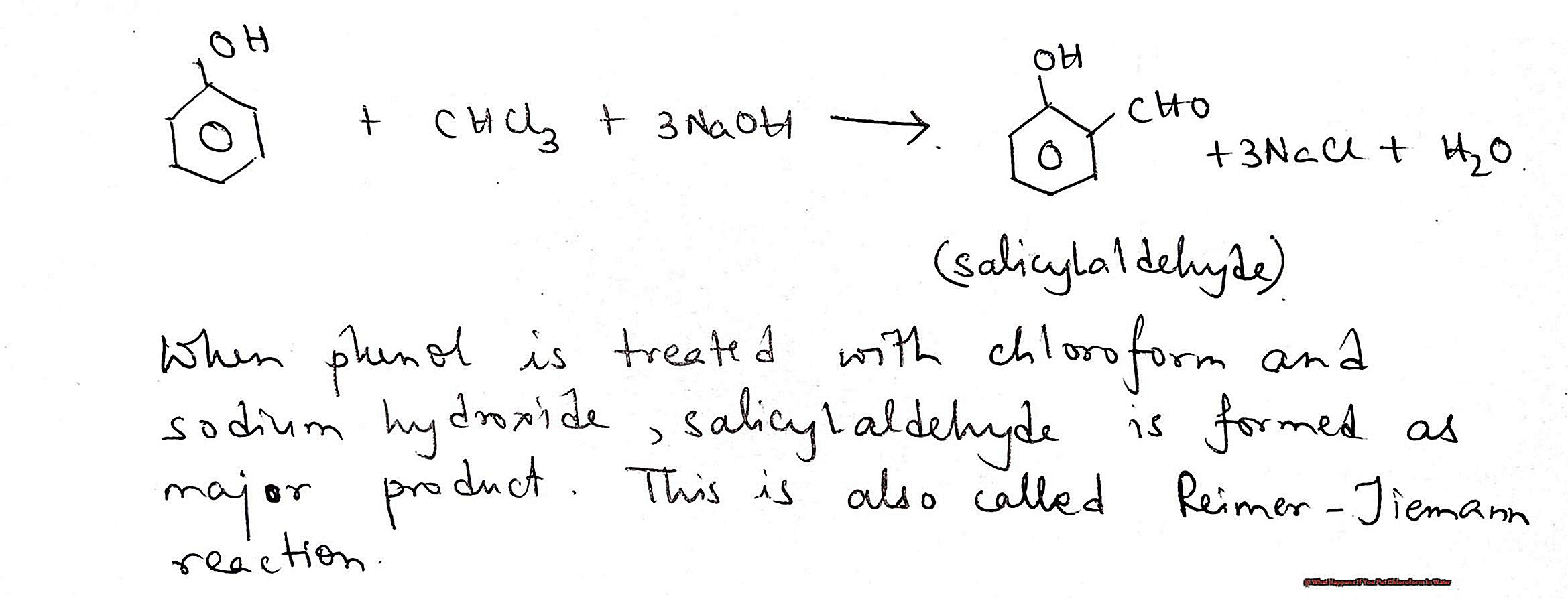
Firstly, let’s talk about what exactly chloroform is. It is a chemical compound with the formula CHCl3 and is commonly used as an anesthetic in medical procedures. However, due to its potential for abuse and harmful effects on health, its use has significantly decreased in recent years.
Now, let’s get to the main question – what happens when chloroform is mixed with water? When chloroform is added to water, it undergoes a process called dissolution, where the molecules of chloroform break down and mix with the molecules of water. This creates a homogeneous mixture, meaning the substances are evenly distributed at a molecular level.
But here’s the catch – chloroform is not very soluble in water. This means that only a small amount of chloroform can dissolve in water at room temperature. The solubility increases with higher temperatures but also depends on the pH level of the water.
So what are the potential risks and concerns associated with this mixture? Firstly, chloroform is considered a volatile organic compound (VOC), meaning it can easily evaporate into the air and contribute to air pollution. It can also contaminate water sources and harm aquatic life once released into the environment.
Moreover, chloroform is classified as a possible carcinogen by the International Agency for Research on Cancer (IARC). Prolonged exposure to high levels of chloroform can lead to respiratory problems, liver and kidney damage, and even neurological issues.
But don’t panic just yet. The good news is that proper disposal methods for chloroform can help prevent any harm to the environment and human health. It is important to handle this chemical compound with caution and follow safety protocols when working with it.
In addition to its effects on the environment and human health, adding chloroform to water can also have an impact on aquatic life. It is toxic to many aquatic organisms and can harm their natural habitats. So if you ever come across any chloroform in water, do not pour it directly into water sources.
The Chemical Properties of Chloroform
Chloroform has been a controversial substance for many years due to its potential health hazards and environmental impact. As an expert on the topic, I am here to shed light on the chemical properties of chloroform, specifically its behavior in water, and provide insights on proper disposal methods.
First and foremost, let’s understand what chloroform is. It is a colorless, volatile liquid with a sweet smell that falls under the category of halogenated organic compounds. This means it contains carbon, hydrogen, and chlorine atoms in its chemical structure. With a molecular formula of CHCl3 and a molecular weight of 119.38 g/mol, chloroform is commonly used as an anesthetic, solvent, and in the production of refrigerants.
However, what happens when chloroform comes in contact with water? The first noticeable change is that the liquid becomes cloudy due to the mixing of the two substances. This is called emulsification, where tiny droplets of chloroform are dispersed in the water. Additionally, chloroform molecules interact with water molecules through hydrogen bonding, resulting in some molecules dissolving in water while others remain as separate droplets.
One important factor to note is that chloroform is highly soluble in water, with a solubility of 8.3 g/100 mL at room temperature. This means that a large amount of chloroform can dissolve in a given volume of water. However, despite its high solubility, chloroform tends to separate from water over time due to differences in molecular structures.
Moreover, chloroform can also react with water molecules to form new compounds. One such reaction is the formation of hydrochloric acid (HCl), which occurs when the chlorine atom in chloroform bonds with a hydrogen atom from a water molecule.
Now, let’s address the elephant in the room – the potential dangers of chloroform. It is considered a toxic substance and has been classified as a possible carcinogen by the International Agency for Research on Cancer. This is why its use has been heavily regulated and restricted in recent years.
The Effects of Adding Chloroform to Water
You may have heard of it as a chemical used in movies to knock someone out, but its uses go beyond Hollywood. This colorless, sweet-smelling liquid has been used for various purposes throughout history, including as an anesthetic and a solvent. However, its use has been heavily restricted due to its potential harmful effects on human health and the environment.
When chloroform is added to water, it can have various effects depending on the concentration and exposure time. In low concentrations, it can cause dizziness, nausea, and vomiting if ingested. It can also irritate the skin and eyes upon contact. But it’s not just short-term exposure that we need to worry about.
In higher concentrations, chloroform can have more severe consequences. It is classified as a probable human carcinogen by the Environmental Protection Agency (EPA), meaning it may cause cancer with long-term exposure. That’s right – this seemingly harmless chemical can increase your risk of developing cancer. It can also affect the central nervous system, liver, and kidneys.
But it’s not just our health that is at risk when it comes to chloroform. One of the main concerns with adding this chemical to water is its potential to contaminate drinking water sources. If it is not properly disposed of or treated, it can enter groundwater and surface water through runoff or leaching from landfills.
And it’s not just a hypothetical risk – according to a study by the EPA, chloroform was detected in 100% of the drinking water samples tested in the United States. While most of these levels were below regulatory limits, long-term exposure to even low levels of chloroform can have adverse health effects.
But humans aren’t the only ones affected by chloroform contamination. The compound is toxic to fish and other aquatic organisms, and can disrupt their reproductive systems and growth. This can lead to imbalances in aquatic ecosystems and harm biodiversity.
So how does this chemical end up in our water? One of the main culprits is improper disposal. Chloroform should never be poured down the drain or thrown in the trash. Instead, it should be taken to a hazardous waste facility where it can be safely treated and disposed of.
Environmental Impact of Chloroform in Water
Chloroform, a colorless and sweet-smelling chemical compound, may seem harmless at first glance. However, its improper disposal and release into water sources can have devastating effects on aquatic life, ecosystems, and human health.
How Does Chloroform Contaminate Water?
There are several ways chloroform can end up in our water sources. Industrial wastewater, runoff from landfills, and improper disposal of household products such as cleaning agents and adhesives are all potential routes for chloroform to enter our water systems. This means that even if we don’t use chloroform directly, we can still contribute to its presence in our water sources through our daily activities.
Harmful Effects on Aquatic Life and Ecosystems
Once chloroform enters the water, it can have severe consequences for aquatic life. Even small concentrations of this chemical can be deadly to fish and other organisms. It can also harm aquatic plants and disturb the balance of the ecosystem in water bodies, leading to a decrease in biodiversity and overall environmental health.
Contamination of Drinking Water Sources
One of the most significant concerns with chloroform in water is its potential to contaminate drinking water sources. If not properly treated, chloroform can enter the public water supply and pose a risk to human health. Long-term exposure to chloroform through drinking water has been linked to an increased risk of certain types of cancer, as well as liver and kidney damage.
Threat to Agriculture
Chloroform in water also poses a threat to agricultural practices. Irrigation using contaminated water can result in crops absorbing the chemical, leading to potential health risks for consumers. It is essential for farmers and communities living near industrial sites to monitor their local water sources for chloroform contamination and take necessary precautions to ensure the safety of their drinking water.
Preventative Measures and Treatment Options
The good news is that we can prevent chloroform from entering our water sources by properly disposing of products containing this chemical. This can be achieved through proper waste management practices and following product disposal instructions carefully.
In cases where chloroform has already contaminated the water, there are treatment methods available. Filtration systems and activated carbon treatment can effectively remove chloroform from water sources, ensuring the safety of our drinking water.
Health Hazards of Chloroform Exposure
Chloroform may have a sweet smell, but don’t let that fool you. This seemingly innocent chemical compound poses serious health hazards for both humans and the environment. From its use as an anesthetic in medical procedures to its production of pesticides and plastics, chloroform is present in various industries. However, it is important to understand the potential dangers of chloroform exposure and take necessary precautions to prevent it from harming ourselves and future generations.
So, what exactly are the health hazards of chloroform exposure? Let’s take a closer look.
How Can Chloroform Enter Our Bodies?
Chloroform can enter our bodies through inhalation, ingestion, or skin contact. Inhalation is the most common route of exposure in water contamination situations. When released into the environment, it can contaminate water sources and break down into toxic byproducts such as dichloromethane and carbon monoxide. Ingestion can also occur if contaminated water is consumed, while skin contact can happen when handling products containing chloroform.
What Are the Short-Term and Long-Term Effects?
Short-term exposure to chloroform can cause symptoms such as dizziness, headache, nausea, and vomiting. Prolonged exposure can lead to more serious health issues such as liver and kidney damage. Pregnant women and fetuses are particularly vulnerable to the effects of chloroform exposure. Studies have shown that high levels of exposure during pregnancy can increase the risk of birth defects and developmental problems in children. Additionally, children are at a higher risk due to their smaller body size and developing organs.
The long-term effects of chloroform exposure are still being studied, but evidence suggests that it may be linked to an increased risk of cancer, specifically liver and kidney cancer. This makes it even more crucial to limit our exposure to this chemical.
How to Protect Yourself and Your Loved Ones?
If you work with or handle chloroform on a regular basis, such as laboratory workers or healthcare professionals, it is important to take necessary precautions to protect yourself. This includes using protective gear such as gloves and masks, working in well-ventilated areas, and following proper disposal methods for products containing chloroform.
Risks for Aquatic Life from Chloroform in Water
Water is essential for all forms of life, and as such, it is our responsibility to ensure its quality and safety. Unfortunately, the presence of chemicals like chloroform in our water sources poses a significant risk to aquatic life. As an expert on this topic, I have seen first-hand the harmful effects of chloroform on marine organisms.
Chloroform is a colorless liquid commonly used as a solvent in various industries. It can enter the ecosystem through industrial waste, agricultural runoff, and water treatment processes. Once it enters the water, it can quickly spread and harm aquatic life through direct contact or inhalation. But what makes chloroform so dangerous for marine organisms?
Studies have shown that even low concentrations of chloroform can be toxic to fish, amphibians, and other marine creatures. It can damage their gills and internal organs, leading to reduced growth and reproduction rates. This not only affects individual organisms but also disrupts the natural balance of ecosystems.
Furthermore, chloroform has the ability to bioaccumulate in the tissues of aquatic organisms. This means that it can build up over time and reach toxic levels, especially for larger organisms that consume smaller ones. This poses a threat not only to aquatic life but also to humans who consume contaminated fish or water.
To protect our water sources and the animals that depend on them, proper disposal and treatment of chloroform-containing waste are crucial. Additionally, regular monitoring of water sources is necessary to ensure levels of chloroform do not reach dangerous levels.
It is also important for industries to adopt more environmentally friendly methods that do not involve the use of chloroform. As consumers, we can also play a role by opting for products that are produced with sustainable practices.
Proper Disposal Methods for Chloroform
Maybe you’ve recently used it for a home project and are now left with the question of how to dispose of it properly. As an expert on proper disposal methods, I’m here to provide you with valuable insights and practical tips for safely and responsibly disposing of chloroform.
First, let’s understand why proper disposal of chloroform is crucial. This colorless liquid may have a sweet smell, but it has been classified by the Environmental Protection Agency (EPA) as a hazardous waste due to its harmful effects on humans and the environment. Pouring it down the drain or into bodies of water not only puts aquatic life at risk but also contaminates our water supply. So what are the right ways to dispose of this hazardous chemical?
The most common method is incineration, where the waste is burned at high temperatures until it turns into ash. This process breaks down the chemicals into less harmful substances, making it a safe option for disposal. However, incineration can be costly and requires specialized facilities.
Another option is chemical treatment, where the waste is mixed with other chemicals to neutralize its toxicity. While this method is effective, it can also be expensive and should only be done by experts.
It is important to note that improper disposal of chloroform can have severe consequences for our ecosystem and communities. This is why it is crucial to contact your local hazardous waste facility for guidance on how to safely package and transport the waste for proper disposal. For larger quantities, it is best to hire a licensed hazardous waste disposal company that has the necessary expertise and equipment.
When handling chloroform for disposal, always wear protective gear such as gloves and goggles to prevent any skin contact or inhalation. It is also essential to store the waste in a tightly sealed container to avoid any spills or leaks.
Proper disposal of chloroform not only protects our health and the environment but also prevents it from falling into the wrong hands. This hazardous chemical can be misused for illegal activities, so it is crucial to dispose of it safely.
Conclusion
In conclusion, the idea of chloroform contaminating our water may sound like a terrifying scenario straight out of a horror film. However, it is not just a fictional concept – there have been instances where chloroform has entered water sources due to various reasons. As we have learned throughout this article, when chloroform comes into contact with water, it can dissolve and form a uniform mixture. But don’t be fooled by its seemingly harmless appearance – chloroform can have severe implications for both our health and the environment.
From its ability to pollute drinking water and harm aquatic life to its classification as a potential carcinogen, proper handling and disposal of chloroform are critical. It is essential to take necessary precautions to prevent its release into the environment and ensure that safe disposal methods are followed.
As consumers, we also have a role to play in protecting our water sources. By choosing products that do not contain chloroform and supporting industries that prioritize eco-friendly practices, we can make a positive impact on the environment. Let’s work together to safeguard our water sources and the creatures that rely on them for survival.





